Accelerate Enrollment + Lower Burden of Execution
ConcertAI’s Digital Trial Solution
What is the Digital Trial Solution?
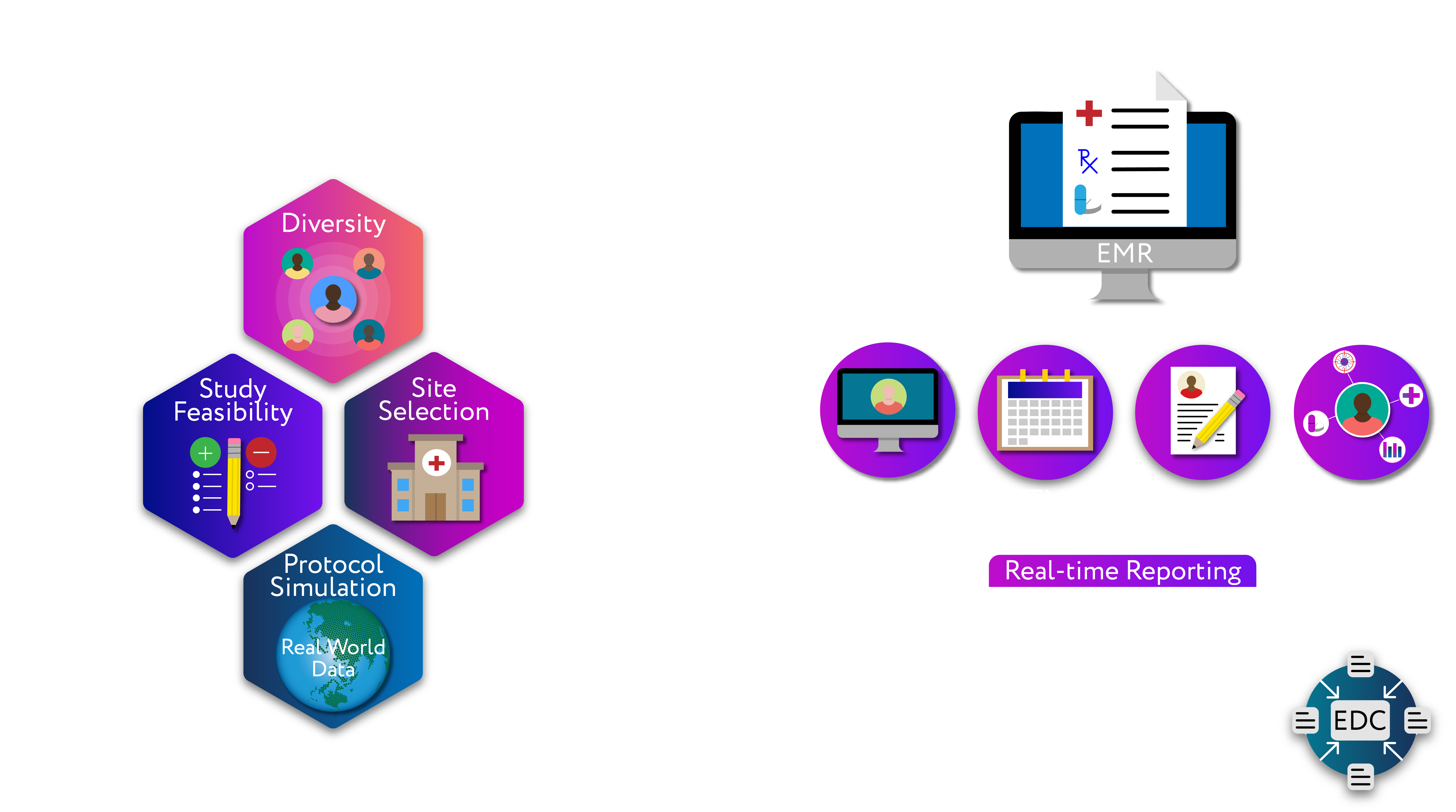
Digital Trial Solution is a hybrid SaaS cloud solution that provides an integrated set of services for clinical trial sites and clinical trial sponsors. It is part of a suite of Digital Clinical Development technologies that extend from study design, protocol optimization, site selection, feasibility assessment, patient trial matching, and eSource (EMR, Molecular, PACS) to eCRF and EDC.
Key Benefits
-
Integrations with research site CTMS for a seamless and efficient solution
-
Real-time matching of patients to clinical trials; workflows guiding eligibility confirmation through to consent
-
Sponsor specific eCRF libraries; intelligent and automated study workflows integrated to research site clinical sources; research team data confirmation workflows with no to minimal data entry; automated through to sponsor EDC
-
Source data provenance and retention of certified facsimiles of clinical data sources
-
Significant study start-up efficiencies; lower clinical operations resource requirements
What Our Clients Say About
The Digital Trial Solution
…Together with ConcertAI, we are fundamentally changing the way we do clinical research from study design, through enrollment and execution…the Digital Trial Solution will allow us to accelerate access to innovative, lifesaving cancer medicines to patients. And we anticipate this approach will become BMS’ gold standard for oncology studies in the future.
– Venkat Sethuraman, SVP Global Biometrics and Data Sciences
Bristol Myers Squibb
…Together with ConcertAI, we are fundamentally changing the way we do clinical research from study design, through enrollment and execution…the Digital Trial Solution will allow us to accelerate access to innovative, lifesaving cancer medicines to patients. And we anticipate this approach will become BMS’ gold standard for oncology studies in the future.
– Venkat Sethuraman, SVP Global Biometrics and Data Sciences
Bristol Myers Squibb
We are extremely proud to have worked with ConcertAI for the past two years to help them shape this highly innovative digital solution…Achieving our first participant enrolled through the Digitally Accelerated Clinical Trial (DACT) model has been a tremendous cross-functional effort and demonstrates BMS’ commitment to use data and technology to simplify and accelerate the clinical research process.
– Marissa Co, VP, R&D Business Insights and Analytics
Bristol Myers Squibb
We are extremely proud to have worked with ConcertAI for the past two years to help them shape this highly innovative digital solution…Achieving our first participant enrolled through the Digitally Accelerated Clinical Trial (DACT) model has been a tremendous cross-functional effort and demonstrates BMS’ commitment to use data and technology to simplify and accelerate the clinical research process.
– Marissa Co, VP, R&D Business Insights and Analytics
Bristol Myers Squibb
Bristol Myers Squibb and ConcertAI Advance Novel Oncology Accelerated Digital Clinical Trial Solution
-
Powered by our RWD, IT infrastructure, digital technology and a broad network of leading research sites
-
Integrated clinical research and practice data simplifies patient identification, informed consent, IRB approval and contract negotiations
-
Researchers are empowered to rethink trial design, recruitment protocol criteria and data collection standards needed to launch more cost-effective, timely and diverse trials
Accelerate Enrollment + Lower Burden of Execution
How it Works