What is Clinical Trial Optimization?
CTO 2.0 is a large-population AI SaaS solution for clinical trial design and optimization in oncology and hematology.
Powered by the largest research data repository covering 900+ providers and research sites.

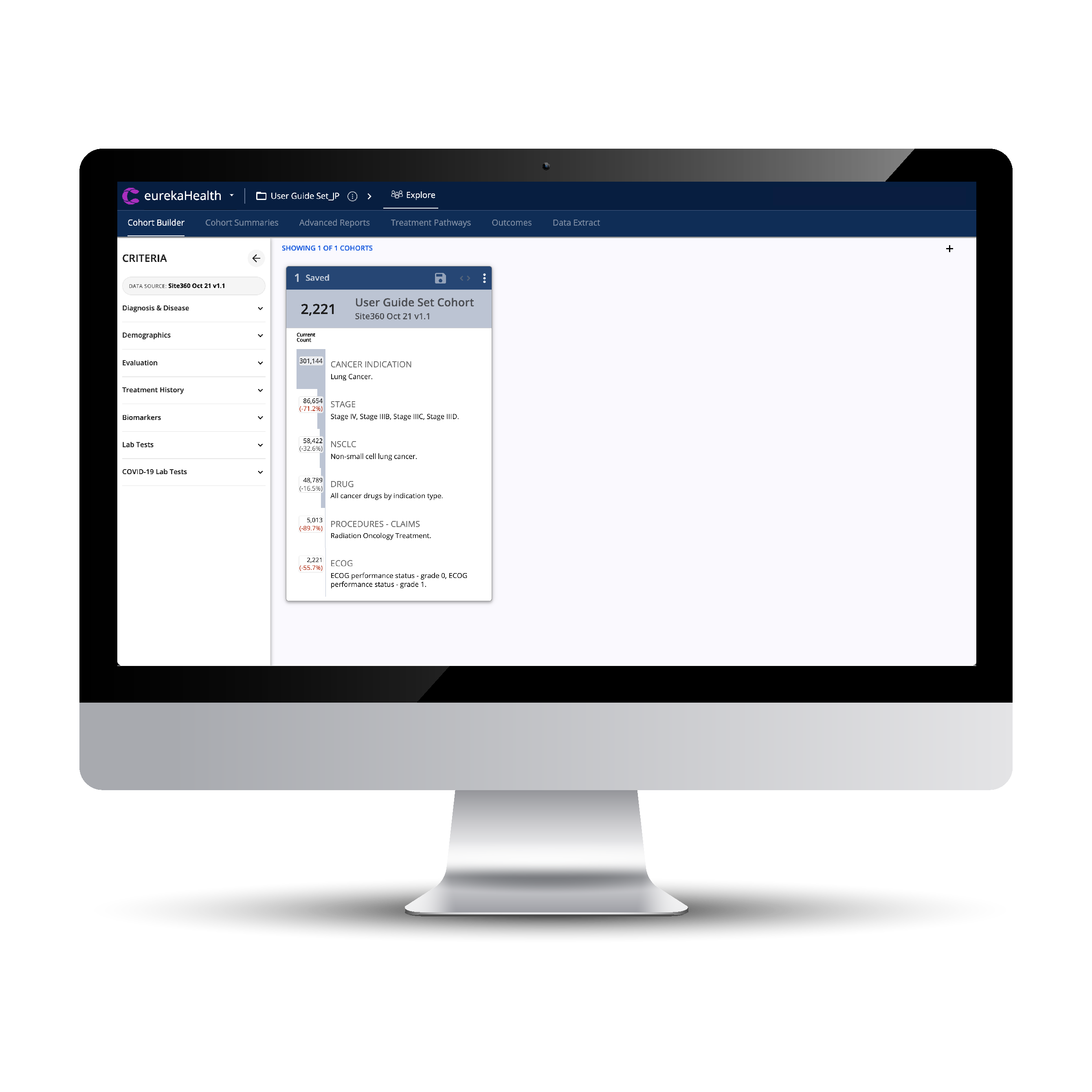
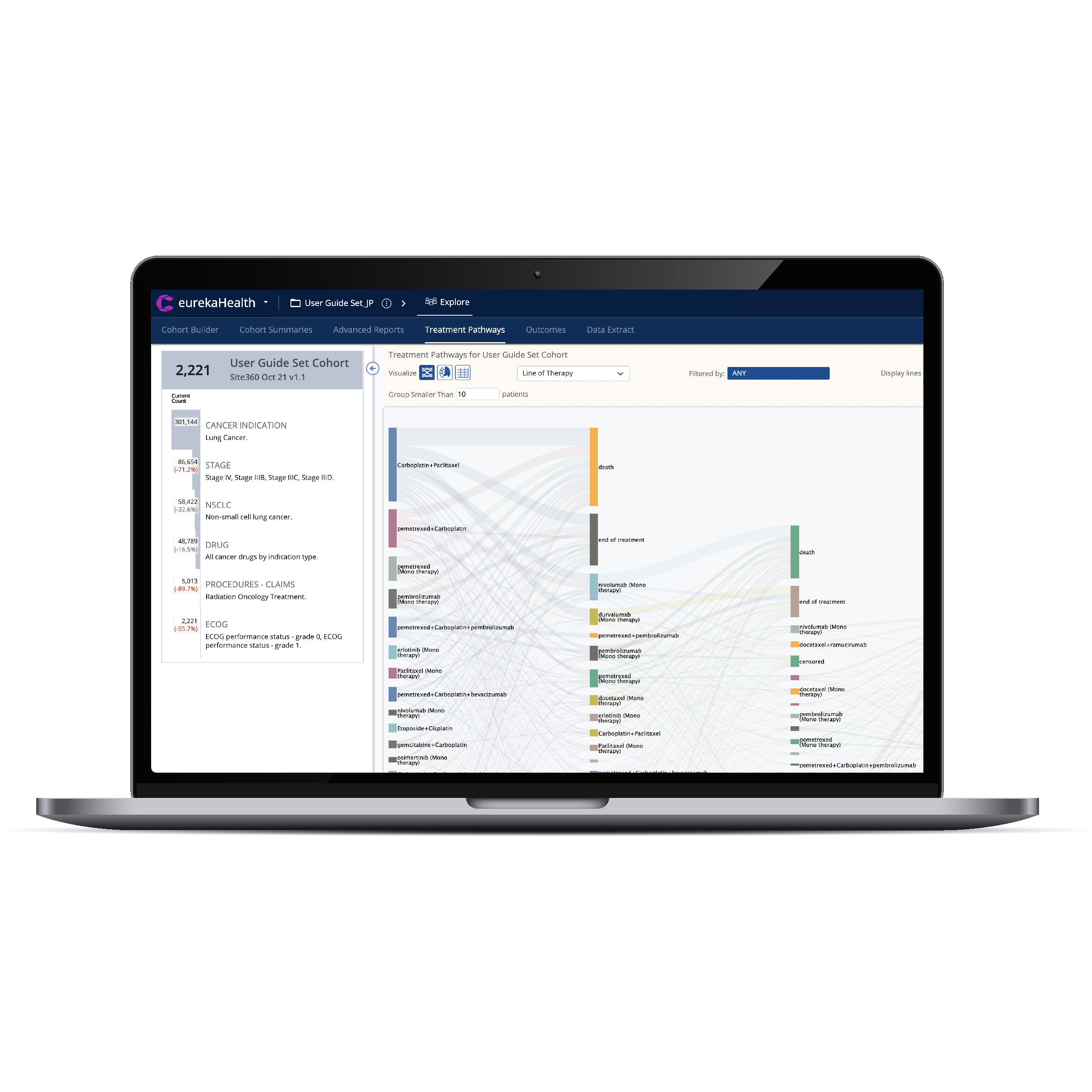
Build and optimize legacy and new studies with unlimited Inclusion and Exclusion criteria.
Cohorts can be assessed with line of therapy tools, Kaplan-Meier Survival Curves, and Cox Proportional Hazard scores.
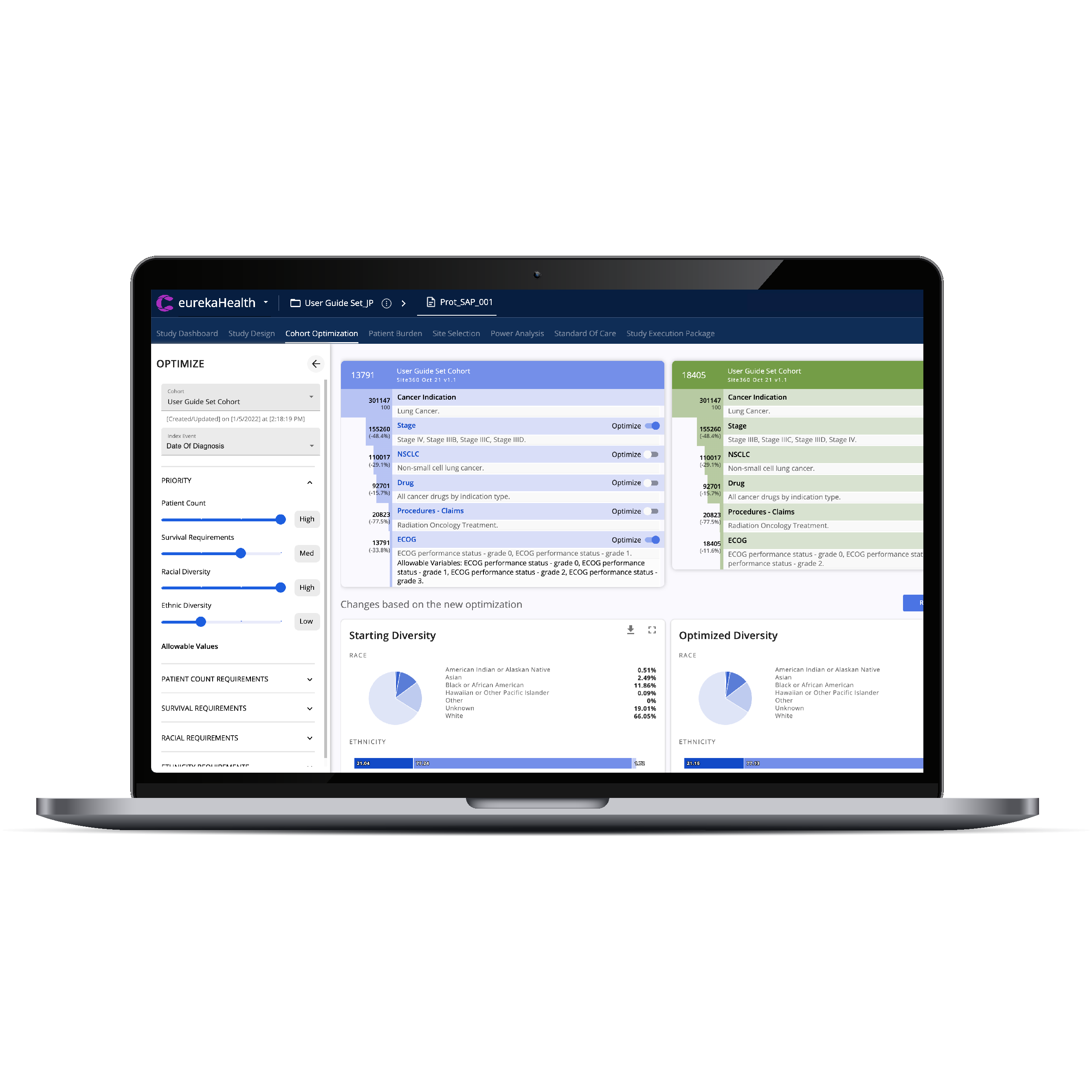
CTO 2.0 is the most advanced solution for trial diversity and study-specific strategies, using EMR and Medical-claims linked to Social Determinants of Health.

High Depth
Clinical Data
THREE-PART INTEGRATED DATA, WITH AN EMR FOUNDATION FOR DIAGNOSIS AND TREATMENTS, LINKED TO MEDICAL CLAIMS AND SOCIAL DETERMINANTS OF HEALTH ASSURE ALL ASPECTS OF POPULATIONS AND SITES CAN BE ASSESSED.

Trial
Diversity
FDA IMPERATIVES FOR TRIAL POPULATIONS TO BE MORE SIMILAR TO THE ULTIMATE TREATED POPULATION AND FOR GREATER DIVERSITY IS ASSURED IN OUR TRIAL MODELING AND AI FOR DESIGNS, COHORTS AND RESEARCH SITES

Research Site
& Patient Burden
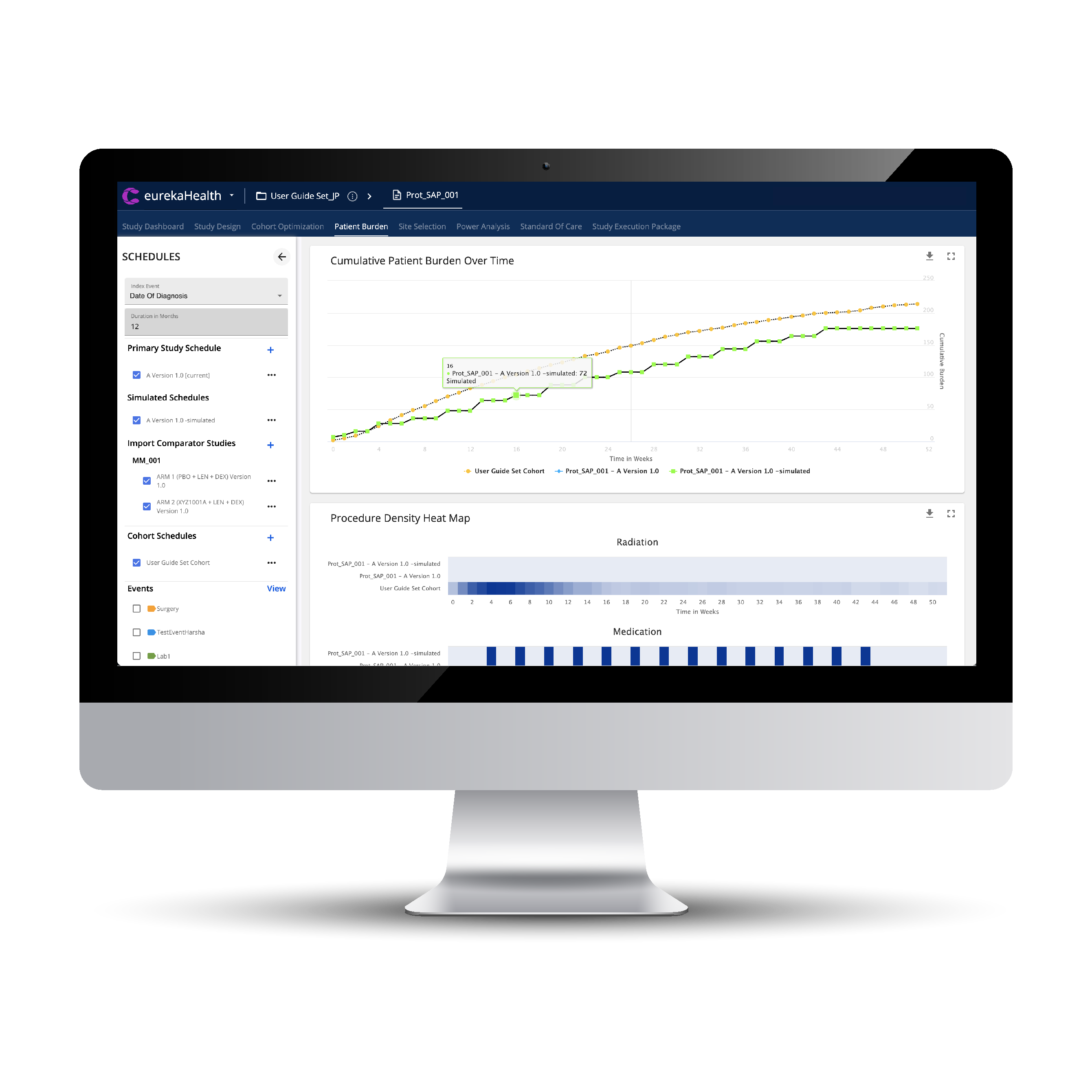
GREATER USE OF RESEARCH CAPABLE COMMUNITY SITES MEANS DESIGNING TO THE LEVEL OF CLINICAL ACTIVITIES OF THE STANDARD OF CARE IN THOSE SETTINGS. OUR BURDEN SCORING AND OPTIMIZATIONS ASSURE THAT.

Biomarkers
CURATED MOLECULAR AND OTHER DATA FROM MULTIPLE SOURCES ASSURE TRIAL MODELING CAN SEE TREATMENT SEQUENCING AND DIAGNOSTIC USE ACROSS SETTINGS.
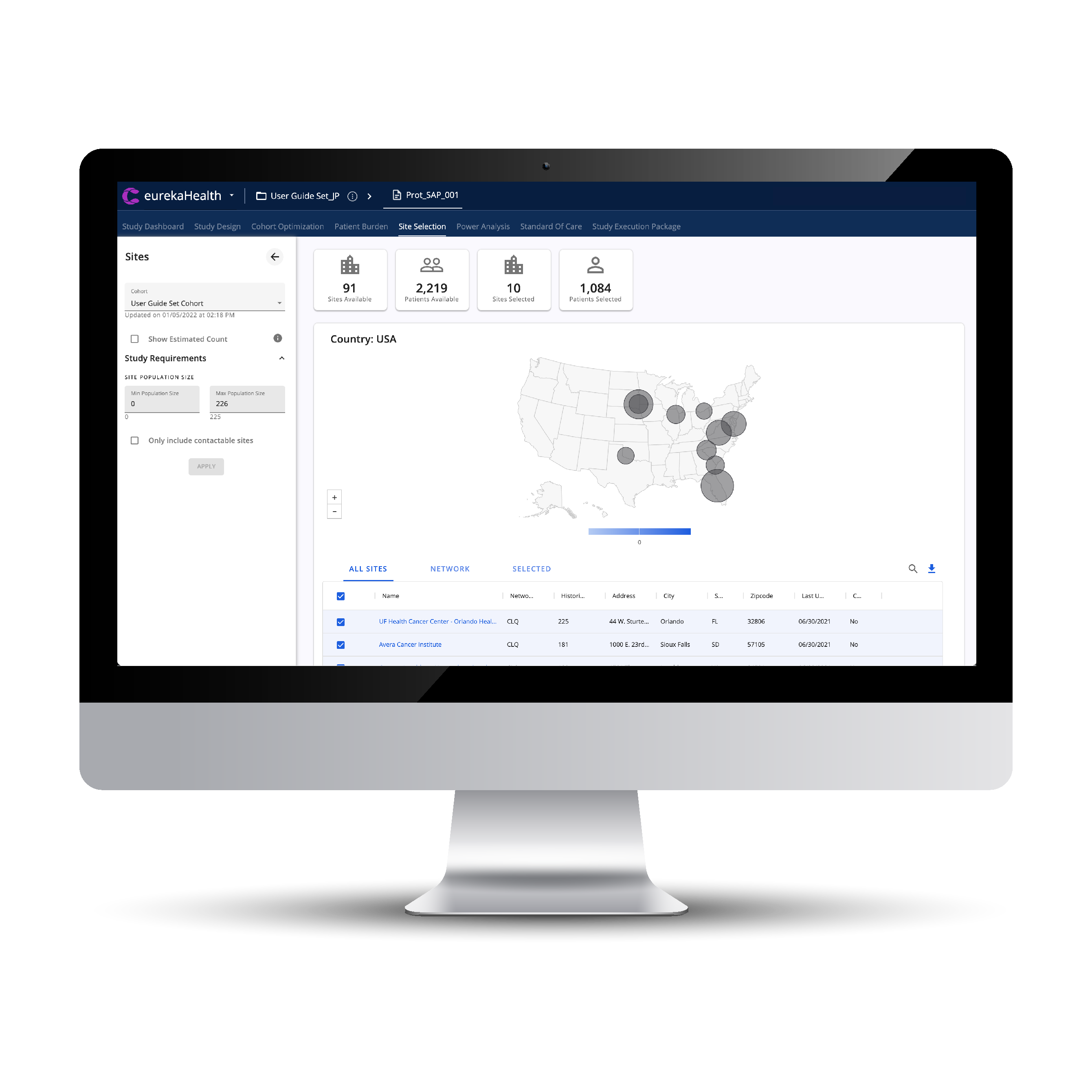
Dynamically Assess
Trial Enrollment Feasibility
and Select Optimal Sites
“Our Feasibility team would stage a coup if we stopped license, in a short time you have become a critical part of our clinical research site selection process”
– CRO Client
“We have our current go-to trial feasibility solution, but discovered that your software and data is a much better fit for our Oncology trials feasibility needs”
– Large Pharma Client
Key Benefits

ASSURE STUDY I/E CRITERIA, OPTIMIZED BURDEN, AND TIGHTLY SYNCHED SITE CAPABILITIES DRIVE PRODUCTIVITY AND TIME BENEFITS

IDENTIFY SITES WITH SPECIFIC ALIGNMENT TO STUDY, IDENTIFYING 30 TO 60% GREATER RESEARCH CAPABLE SITES THAN WERE USED IN LEGACY APPROACHES

BUILD TRIAL GENERALIZABILITY AND DIVERSITY STRATEGIES AND IMPLEMENT THESE AT THE PROGRAM, STUDY AND SITE LEVELS.
.svg)