By Elizabeth Sweeney, PhD, ConcertAI and
Samuel Heilbroner, MD, ConcertAI
The Covid-19 pandemic has had several adverse impacts on the practice of oncology, including delays in diagnosis, alterations to patient care strategies, and concerns with safe access to infusion clinics for patients especially vulnerable to a CoV-2 infection. These issues have gone well beyond the practice of medicine and into the clinical trials arena.
In 2020, approximately 280 oncology clinical trials were impacted by the pandemic via extended delays or by suspension of the trial1. This has not only obstructed the process by which pharmaceutical companies get new and effective therapies out to the cancer community, but has also negatively affected access to clinical trials and novel therapeutics for cancer patients, both currently enrolled in a clinical trial and those who planned to participate in a trial. The restrictions associated with the Covid-19 pandemic have resulted in difficulties with successful participant enrollment and with maintenance of existing participants on the approved trial protocol, causing difficulties in data interpretability and successful completion of the trial.
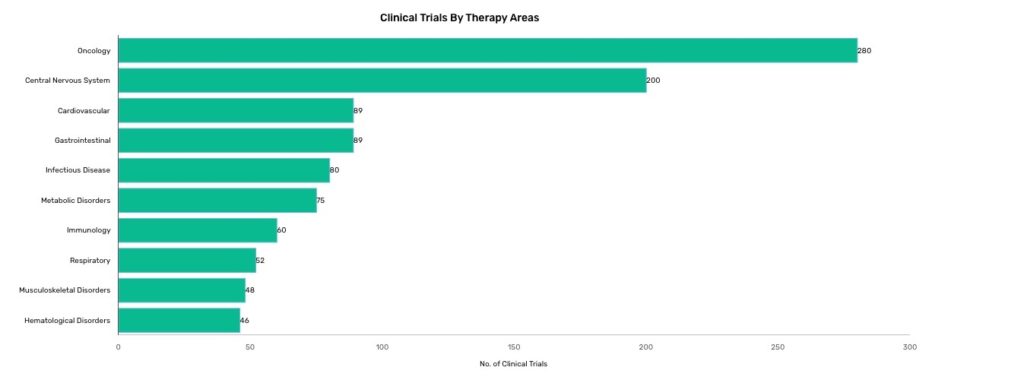 Source: GlobalData Distrupted Clinical Trials dashboard (January 20, 2021)
Source: GlobalData Distrupted Clinical Trials dashboard (January 20, 2021)
Trial enrollment issues are not a new phenomenon. Several studies have noted oncology clinical trial participation is typically low, between 5-9%, and trials regularly report a participant dropout rate of ~30%2,3. Further, diversity recruitment barriers have been documented for several years, including low enrollment of underserved cancer patients, limited race/ethnicity variability of participants, and inadequate inclusion of cancer patients from rural communities.
Although reduction in enrollment success due to the Covid-19 pandemic has added to this ongoing complication for both study design and successful execution of clinical trials, it has also spurred changes to the regulations around clinical trial design that will positively affect the oncology field in the future. Specifically, the FDA approved temporary policies during the pandemic involving the use of real-world data (RWD) and technology solutions for both study design and site selection which could be permanently incorporated into the governance of clinical trial planning in the near future.
As a result of these seismic shifts in healthcare, and the need to stem and reverse the declines from Covid-19, there is a digital revolution underway in life sciences companies that will be long-lasting. The ability to use AI, cloud, and SaaS technologies to drive insights and evidence from RWD to optimize for patient enrollment is bringing about innovation and new ways of thinking needed to account for and understand the changes due to COVID-19.
The use of digital platforms linked to large databases of patient RWD coupled with advanced analytics to expand clinical trial eligibility criteria and site selection options will support many novel approaches to study design and site selection:
- Maximize and accelerate patient enrollment Digital solutions will have the ability to capture, standardize, and integrate vast amounts of patient specific information from multiple different sources such as clinical records, genomic testing, and claims data. .
By leveraging these types of different datasets within one technology environment, it is possible to derive a more complete picture of the patient. Recent advances in AI and ML can help researchers optimize for criteria that increases the patient pool. Further, once trial design is complete, AI technologies can rapidly identify candidate trial participants, calculate the probability that they will meet enrollment criteria, and streamline the screening process. This will help researchers better target and more confidently identify eligible candidates for clinical trials. - Enhanced trial site footprint. The increased adoption of digital solutions will enable researchers to identify new, previously under-represented sites and enable their participation in clinical trials. Quality site selection is an important factor in offsetting enrollment delays and low enrollment issues for clinical trial planning. By linking site information from RWD to clinical trials databases like ClinicalTrials.gov, researchers can easily identify sites that have performed well in previous clinical trials.
Additionally, RWD can identify sites with the potential for high minority enrollment and flag trial criteria that are likely to limit minority participation. Studies can be designed digitally, allowing researchers to test scenarios before starting the trial and see the impact of design decisions on diversity in real time.
By supporting faster patient accrual and enlisting more sites with eligible patients, clinical researchers will make clinical trials more inclusive and more successful.
ConcertAI is the leading partner for clinical researchers using advanced AI and ML to quickly assess, adjust and modify trial designs – for optimal patient enrollment. Contact us today to learn more about how our enterprise clinical development solutions can help your program!
Sources
1 GlobalData Distrupted Clinical Trials dashboard (January 20, 2021)
2 “Clinical Trials: The Art of Enrollment,” Seminars in Oncology Nursing, November 2008, Pages 262-269
3 “The Future of Clinical Trials,” Everest Group, 2018



